The Pharma Potential to Tap into Biobanks
George Dagher, PhD, coordinator of the French infrastructure for biobanks and leader of funding and financing WP, Biobanking and Biomolecular Resources Research Infrastructure (BBMRI) shares some thoughts with Lina Genovesi on the opportunities of biobanks for pharma.

From Dagher’s perspective, “for pharma to seize the opportunities of biobanks, the reliable accessibility to biological specimens is key. This accessibility may be provided by a common platform or infrastructure regrouping of different biobanks to ensure that biological specimens are made available in a consistent and reliable way and where the sharing of specimens and data is regulated. These biobanks may systematically be integrated into healthcare systems and may be the essential foundation for the future.”
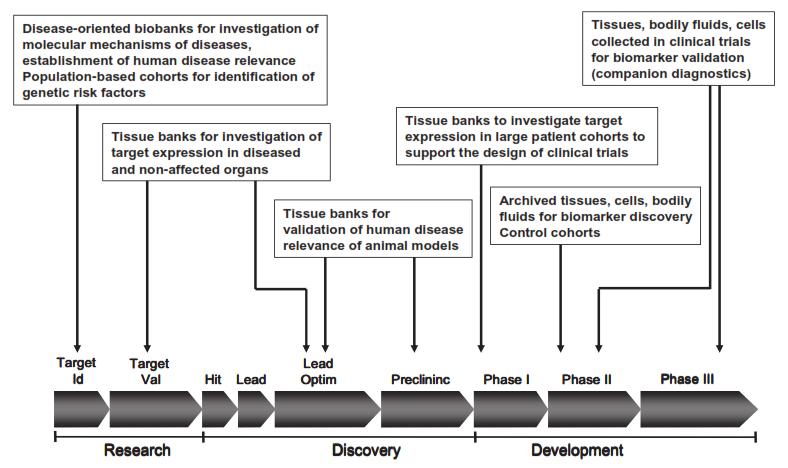
“Pharma turns to these biological samples for biomarkers. These biomarkers are incorporated into a biomarker strategy for target identification, lead optimization, preclinical and clinical development to shorten the drug development timeline, enhance time to market while reducing the overall drug development costs.”
Source: BBMRI Business Plan 03/12/2012. Click to enlarge.
“These biomarkers have specific applicability to cancer-targeted drug development and treatment. They allow the stratification of a population on the basis of a specific “genotype” associated with a disease rather than relying on a report of the “family history” of the disease. The ability to quantify “susceptibility” in this way could be an extremely important method for estimating genetic susceptibility and disease risk to a specific cancer among various populations.”
The need of pharma for biomarkers is not expected to wane any time soon. According to a Biomarkers Market research report (M&M, Texas, January 2012), the global biomarker market is growing at a steady pace of Compound Annual Growth Rate (CAGR) of 14.40% to reach $25.79 billion by 2016, driven by the demand in for biomarkers in drug discovery. Oncology is seen to be the largest indication in terms of revenue with cardiology the fastest growing indication with a CAGR of 16.24% from 2011 to 2016.
“With such expected growth, it is imperative that a platform is provided,” states Dagher. “Such platform is exemplified by the European Biobanking and Biomolecular Resources Research Infrastructure (BBMRI). BBMRI aims to improve the accessibility and interoperability of biological samples from different (sub)-populations of Europe. These collections also include data on factors such as health status, nutrition, lifestyle, and environmental exposure of study subjects and are part of a Europe-wide platform for translational medical research to develop personalized medicine and disease prevention.”
“This platform builds on existing sample collections and biobanking technologies. All resources are integrated into a pan-European distributed hub-and-spoke structure-like network and embedded into European scientific, ethical, legal and societal frameworks. Interoperability is achieved by implementation of common standards and access rules which consider the heterogeneity of pertinent national legislation and ethical principles.”
From Dagher’s perspective, there are special issues to be considered for accessing biological samples by pharma. These issues revolve around balancing the public interest of sharing samples and data and the interest of pharma of protecting innovations to maintain market share.
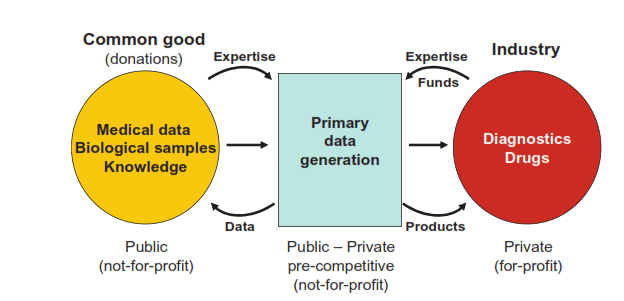
“With these issues in mind, BBMRI developed the concept of expert centers as a new model for public-private partnerships.”
Source: BBMRI Business Plan 03/12/2012. Click to enlarge.
“These centers are responsible for the analysis of samples in the country of origin under internationally standardized conditions and the generation of primary data. They integrate pre- competitive public and private research and development activities by providing not only access to biological samples and medical data but also to the broad spectrum of medical and scientific expertise related to the samples, data, and their analysis.”
“These centers create “a win-win” situation by enhancing collaborative research, using limited resources efficiently, sharing data, technology, knowledge and expertise, - facilitating innovation, increasing competitiveness in academic science as well as on the marketplace through product innovation and increased R&D efficacy.”
To tap into the power of biobanks, Dagher states unequivocally that funding is key for the sustainability of biobanks. “Most conventional grant mechanisms with limited periods of funding are not sufficient for maintaining the sustainability of biobanks and we need to devise creative funding mechanisms such as nesting biobanks in the health-care system. To support this nesting requires the development of a regulatory framework, mechanisms for quality management, and data storage, transfer, and integration which are all harmonized and aligned to meet current and future needs. “
“Nesting these biobanks into healthcare systems may be the essential foundation for the future” concludes Dagher.